Defining What Makes a Fluid a Fluid
When you think of the word "fluid" what comes to mind? Many of us might instantly think of liquids like water, juice, or milk, while others might think of gases like air or steam. However, the concept of "fluid" is much broader than that. In this article, we'll explore the definition of fluids, their properties and types, and how they exist all around us in our everyday lives.
What is a Fluid?
Put simply, a fluid is any substance that can flow and also take the shape of its container. Fluids include both liquids and gases, as well as substances that can exhibit both properties, such as plasma.
The key characteristic that defines a fluid is its ability to change shape without a specific limit, unlike solids which have a fixed shape and volume. The video below, from researches at MIT studying coating processes, is a perfect example to help build your intuitive understanding of the definition of a fluid.
Fluids are incredibly important in our everyday lives. For example, water is a fluid that we rely on for hydration, cleaning, and cooking. Blood is another important fluid in our bodies that helps transport nutrients, oxygen, and waste products throughout our circulatory system. Fluids also play a crucial role in industries such as engineering and manufacturing.
It's important to note that not all fluids are created equal. Some fluids, such as honey or molasses, are more viscous and flow more slowly than others. Meanwhile, gases like helium or nitrogen are much less dense than liquids and can expand to fill their containers.
Despite their differences, all fluids share a common characteristic: they can flow and change shape without a specific limit. This makes them incredibly versatile and useful in a wide range of applications.
What Are Fluid Properties?
Fluids have unique physical properties that govern their behavior and movement. These physical properties are essential for understanding the behavior of fluids in various applications, including engineering, physics, and chemistry. The three primary physical properties are its density, its viscosity and its surface tension. Fluids also derive additional thermodynamic properties from their environment.
What is Density?
Density is a physical property that defines its mass per unit volume. The density of a fluid is influenced by factors such as temperature, pressure, and the composition of the fluid.
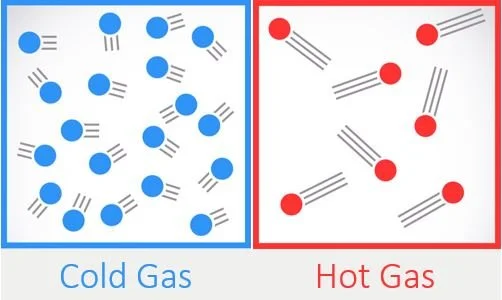
For example, when a liquid is heated the molecules become more energetic, moving faster and crashing into each other more frequently. This leads to the molecules spreading apart to a larger degree and taking up more space per molecule. They become less dense. Of course the opposite occurs when a liquid or gas is cooled. In this molecular sense, density can be thought of as the number of molecules occupying a set volume. As can be seen in the image below, the colder gas has fewer molecules in the given volume, making it more dense.
Differences in density give rise to relative motions of fluids submersed within one another, will less dense fluids rising and more dense fluids sinking. This effect is termed buoyancy.
What is Viscosity?
Viscosity is the degree to which internal friction between particles in a fluid resists its movement. Viscosity is influenced by factors such as temperature, pressure, and the composition of the fluid.
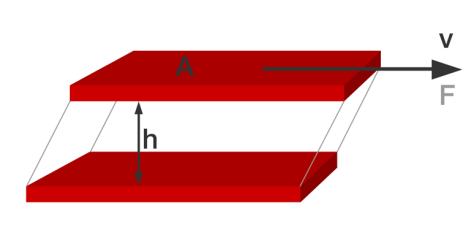
For example, honey has a higher viscosity than water, explaining why it pours from a bottle more slowly. The most intuitive mental image defining the viscosity of a fluid is provided by the shear test typically used to measure a Newtonian fluid’s viscosity. Newtonian fluids are the most common types of fluids and are characterized by the fact that the rate of flow (strain rate) is linearly proportional to the for (shear stress) applied. And the factor of proportionality is the viscosity of the fluid.
In this test a fluid is trapped between a moving plate and a static plate at a known separation distance, as shown in the image below. The force required to move the moving plate is measured. The viscosity can then be calculated (again, for Newtonian fluids only) as the force (F) times the fluid height (h) divided by the Area of the plate (A) times the Velocity of the moving plate (V). We will save the discussion of non-Newtonian fluids for another article.
The viscosity of a fluid is typically given in the relatively strange unit of mass per unit length per unit time, with the most common unit being a Poise, or a g/cm-s.
What is Surface Tension?
Surface tension is a physical fluid property of liquids that arises due to the cohesive forces between molecules at a liquid's surface. These cohesive forces are the result of the attractive forces between the liquid's molecules. At the surface of the liquid, the molecules are not surrounded by other liquid molecules on all sides. This imbalance of forces causes the fluid to behave as if it is covered with a thin elastic film.
The unit of surface tension is force per unit length, typically expressed in dynes per centimeter (dyn/cm) or millinewtons per meter (mN/m). Surface tension depends on factors like the type of liquid, temperature, and the presence of impurities or surfactants that can reduce surface tension.
Surface tension can be observed in various phenomena, such as the formation of drops, the ability of some insects to "walk" on water, and the tendency of a liquid to minimize its surface area (e.g., a liquid droplet forming a spherical shape).
Thermodynamic Properties
Additionally, fluids take on thermodynamic properties depending upon their state. These properties include most commonly temperature and pressure. In layman’s terms, temperature is how hot or cold something is, though in gas thermodynamics it has a very specific definition relating to the mean kinetic energy of the molecules. Temperature is measured in either Kelvin, Celsius or Fahrenheit. On the other hand, pressure is simply defined as the force per unit area that the fluid exerts on its container with the respective units of Force divided by Area. Pressure can vary depending on factors such as depth, altitude, and the volume of the fluid. For example, the pressure at the bottom of a swimming pool is greater than the pressure at the surface due to the weight of the water above.
Overall, fluid properties are essential for understanding their behavior and movement in various applications. By studying these properties, scientists and engineers can develop new technologies and improve existing ones to benefit society.
What are the Different Types of Fluids?
There are two main types or classes of fluids: liquids and gases. There are additional special fluids can exhibit both properties, such as plasmas and superfluids.
What is a Liquid?
Liquids are characterized by having a fixed volume but no fixed shape. This means that they take the shape of their container. For example, when you pour water into a cup, the water takes the shape of the cup. Liquids can also exhibit surface tension, which arises from the attraction of particles at a liquid-gas interface. This is why water droplets form beads on surfaces or why insects can walk on water without sinking.
What is a Gas?
Gases, on the other hand, have no fixed volume or shape. They will expand to fill any available space. For example, when you open a can of soda, the carbon dioxide gas escapes and fills the space around it. Gases are also highly compressible, which means that they can be squeezed into a smaller volume. This is why gas tanks can hold a large amount of compressed gas. Gases are typically less dense than fluids causing them to display buoyancy.
Special Fluids
There are also special cases of fluids such as plasmas and superfluids. Plasmas are ionized gases that exhibit unique properties such as electrical conductivity. They are found in lightning, stars, and some types of flames. Another example of a special fluid is superfluids. Superfluids have zero viscosity and can flow indefinitely without friction. They are used in applications such as cooling superconducting magnets and studying quantum mechanics.
Fluids in Nature
Fluids exist all around us, with by far the two most common being from the water we drink and the air we breathe.
In nature, fluids also have a significant impact on our environment. Perhaps most important of all is the hydrologic (water) cycle process, which involves the continuous movement of water, in liquid, gas, and solid phases, through the atmosphere, land, and oceans. This constant movement of fluids also helps to regulate the Earth's climate and temperature. The water cycle is essential for the survival of all living organisms. The oceans, which cover over 70% of the Earth's surface, are also a vital liquid reservoir that supports diverse marine life and plays a crucial role in regulating the Earth's climate.
Did you know that the human body is made up of approximately 60% water? That's right, we are essentially walking bags of fluid! Water is essential for our bodies to function properly, as it helps to regulate body temperature, transport nutrients, and remove waste.
Fluid dynamics of winged flight
Fluids in Technology
In addition to natural phenomena and the human body, fluids also play essential roles in various industries. For example, in aviation, fluids such as jet fuel and hydraulic fluid are used to power and control aircraft. Heating and cooling systems in buildings also rely on fluids, such as water and refrigerants, to regulate temperature. Even cosmetics, such as lotions and creams, contain fluids that help to moisturize and protect the skin.
The study of fluids (fluid mechanics) and their behavior (fluid dynamics) has led to the development of many technologies that we rely on every day. For instance, fluid lubricants help reduce friction and wear between moving parts. Pumps, for example, are used to move fluids from one place to another, such as pumping water from a well. Turbines, on the other hand, use the motion of fluids to generate electricity, such as in hydroelectric power plants. Hydraulics, which involves the use of fluids to transmit power and control machinery, is used in everything from car brakes to construction equipment. The operation of a pumps is demonstrated in the CFD model animation below.
Computational Fluid Dynamics model of a pump
Fluids in Everyday Life
We encounter fluids in our daily lives in a multitude of ways, whether we're cooking, cleaning, or simply drinking water. Many popular beverages like soda and coffee are examples of liquids, while the air we breathe is composed mainly of gases like oxygen and nitrogen.
Other examples of fluids in our everyday lives can include taking a shower, where water flows over our bodies and helps to remove dirt and sweat. Our cars depend on fluids such as gasoline or motor oil to function and stay lubricated. In essence, fluids are essential and omnipresent in our lives.
Moreover, fluids have fascinated scientists and engineers for centuries. The study of fluids, known as fluid mechanics, has contributed to many groundbreaking discoveries and inventions. For example, the principles of fluid mechanics are used in designing efficient airplane wings and in understanding how blood flows through our arteries and veins.
Understanding the properties and behavior of fluids (fluid dynamics) is essential for solving many real-world problems and advancing our knowledge of the world around us, and the reason so much research, such as that from a study of winged flight shown below, is devoted to fluids.
What is the Difference between Liquids and Gases?
While both liquids and gases are types of fluids, there are significant differences between them. One of the most notable differences is their compressibility, which refers to how much their volume can change under pressure. Gases are highly compressible and can occupy any available space, while liquids are less compressible and have a relatively fixed volume.
Another difference is surface tension, which is a property unique to liquids. This arises because liquid molecules, unlike gas molecules, have a mutual attraction that creates a surface tension at the air-liquid interface. Gases do not have surface tension, since their molecules are not held together in the same way.
Furthermore, liquids and gases have different densities. The density of a substance is defined as the amount of mass per unit volume. Liquids are generally denser than gases, which means that they have more mass per unit volume. For example, water is much denser than air, which is why it sinks in air and vice versa. Water vapor rising from the Excelsior Geyser is a result of steam having a lower density than air. This effect of fluids segregating themselves by density is called buoyancy. The buoyancy effect has about a gazillion implications in the real world and shall have its own article.
Another important difference between liquids and gases is their behavior under different temperatures and pressures. Liquids tend to maintain their volume and shape under normal conditions, while gases tend to expand and fill any available space. However, when liquids are heated, they can expand and become less dense, while gases can be compressed and become more dense.
Additionally, liquids and gases have different properties when it comes to flow. Liquids flow easily and can be poured, while gases need to be contained in a closed system in order to be directed or controlled. This is because liquids have a fixed volume and shape, while gases can expand and fill any available space.
Finally, liquids and gases have different properties when it comes to their interactions with other substances. Liquids can dissolve other substances, while gases can only dissolve in liquids. This is because liquids have a fixed volume and shape, while gases can expand and fill any available space.
Conclusions
From liquids to gases, fluids play an essential role in our world and in various fields of study. Understanding the properties and behavior of fluids can help us to design better technologies, study natural phenomena, and improve our daily lives. So the next time you take a sip of water or breathe in the air around you, remember that you are interacting with some of the most fascinating substances known to science.